Ionization Energy
The ionization energy , represented by the symbol I is the minimum amount of energy required to tear the outermost electron to one mole of atoms in a gaseous state , a ground state. In other words, it is the minimum amount of energy required to transform a mole of neutral atoms in a gaseous state into a mole of ions with a positive charge (hence its name).
Seen in the form of a chemical equation, the ionization energy would be the energy required for the following process:
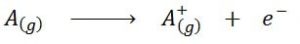
Ionization energy is a direct measure of how tightly the outermost valence electrons are bound to an atom of a chemical element. By being defined as the energy of the ionization process in the gaseous state, the contribution or interference of intermolecular interactions that occur in the liquid and solid states is avoided.
In this way, it is ensured that the ionization energy only depends on the internal forces of the atom and, in particular, on the stability of the electrons that form the valence shell of each element.
The process of removing an electron from the valence shell is a process that requires energy, so it is an endothermic process. For this reason, ionization energies are always positive (by convention, when energy enters a system it is considered positive).
There is more than one ionization energy
Although the definition of ionization energy applies to neutral atoms that are converted to positive ions (i.e. cations), it can also be applied to the successive removal of electrons from positive ions, i.e. species that already they have lost electrons.
In this sense, the energy to ionize the neutral atom is only the first of many possible ionization energies, since there is one for each electron that revolves around the nucleus.
In other words, the energies associated with all of the following processes are considered ionization energies:

Order of successive ionization energies
For any atom of any element, it is true that an ionization energy will always be greater than all previous ionization energies. In other words, the successive ionization energies have the following relationship:
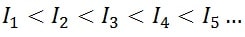
The reason why this happens is very simple. The first ionization energy involves removing an electron from a neutral atom. However, the second ionization energy involves removing an electron from an atom that has already lost the first.
The result is that it is more difficult to start the second electron than the first, and it will be more difficult to start the third than the second, and so on, as can be seen in the following table of ionization energies for the first 10 elements of the table periodic.
|
Z |
Element |
1st |
2nd |
3rd |
4th |
5th |
6th |
|
1 |
H |
1312 |
|
|
|
|
|
|
2 |
He |
2373 |
5251 |
|
|
|
|
|
3 |
Li |
520 |
7300 |
11815 |
|
|
|
|
4 |
Be |
899 |
1757 |
14850 |
21005 |
|
|
|
5 |
B |
801 |
2430 |
3660 |
25000 |
32820 |
|
|
6 |
C |
1086 |
2350 |
4620 |
6220 |
38000 |
47261 |
|
7 |
N |
1400 |
2860 |
4580 |
7500 |
9400 |
53000 |
|
8 |
O |
1314 |
3390 |
5300 |
7470 |
11000 |
13000 |
|
9 |
F |
1680 |
3370 |
6050 |
8400 |
11000 |
15200 |
|
10 |
Ne |
2080 |
3950 |
6120 |
9370 |
12200 |
15000 |
Periodic trend of ionization energy
Ionization energy is a periodic property that increases from left to right and from bottom to top in the periodic table, as can be seen in the following graph .
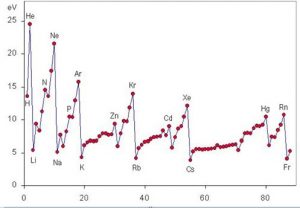
Variation of I over a period
As we move over a period from left to right, something called the effective nuclear charge progressively increases, which is nothing more than the actual positive charge that the outermost electrons can “see” due to the shielding of the innermost electrons.
This means that the force with which the nucleus is able to attract the valence electrons increases, making it more difficult to start them and the energy required (that is, the ionization energy) becomes greater.
Variation of I across a group
By descending into a group, we are placing electrons in ever-increasing energy levels, and thus in atomic orbitals ever further from the nucleus. For this reason, the force with which the nucleus attracts the valence electrons decreases as we go down in a group, thus decreasing the ionization energy.
How to determine ionization energy?
Ionization energy is a quantity determined experimentally by means of a series of techniques collectively called photoelectron spectroscopy.
These techniques are based on a phenomenon very similar to the photoelectric effect, in which electromagnetic radiation is capable of removing electrons from an atom, and the difference between the energy of the radiation and the kinetic energy with which the electron is fired represents the ionization energy.
The different photoelectron spectroscopy techniques allow us to analyze the energies with which practically any electron is bound to its nucleus, be it a valence electron or an internal electron.
Examples of ionization energy
First energies of ionization of noble gases
Noble gases have the most stable electronic configurations of all the elements in the periodic table. For this reason, they also have the highest ionization energies. The first ionization energy of each of the noble gases is presented below:
-
Helium
Its first ionization energy is 2373 kJ / mol, the highest in the entire periodic table.
-
Neon
Its first ionization energy is 2080 kJ / mol and it is the second highest.
-
Argon
Its first ionization energy is 1521 kJ / mol. Only F, Ne and He have higher ionization energies.
-
Krypton
The first ionization energy is 1350 kJ / mol. It is not as high as the others, but it is still higher than that of its neighboring elements.
-
Xenon
The same is said of krypton can be said of xenon with its first ionization energy of 1170 kJ / mol.
First and second ionization energies of alkali metals
The alkali metals in turn have the lowest first ionization energy and the highest second ionization energy of all the elements:
-
Lithium
Its first ionization energy is less than a quarter that of he, but lithium has the second highest ionization energy of all elements, which is 7300 kJ / mol.
-
Sodium
Sodium very easily loses its first electron, since with this it acquires the electronic configuration of Ne, but to eliminate the second electron, 4560kJ / mol must be supplied.
-
Potassium
The first ionization energy of potassium is only 418.7 kJ / mol, while the second is 3052 kJ / mol, considerably higher than that of its neighbors.
-
Rubidium
With an ionization energy of 403 kJ / mol, rubidium is one of the elements with the lowest ionization energy. However, the second is 2633 kJ / mol.
-
Cesium
Its first ionization energy is only 375 kJ / mol and the second 2234 kJ / mol, even less than the first ionization energy of helium.